Darren W. Johnson Laboratory at the University of Oregon
PN-Heterocycles
Collaboration with the Haley Lab
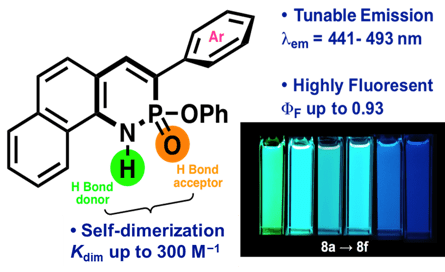
The Azaphosphinine project centers on synthesizing and analyzing a specific class of 1,2-λ5 phosphorus and nitrogen heterocycles, extensively studied since their discovery in 2015 and with a particular focus on supramolecular association and photophysical properties. Supramolecular association arises from the interaction between the strong hydrogen-bond accepting phosphonyl group and the hydrogen-bond donating N–H, measurable by 1H and 31P NMR, with adjustability through pendant functional groups. Research also delves into incorporating a second hydrogen-bond acceptor via a pyridine ring, inducing unexpected tautomerization leading to a quinoidal, non-aromatic configuration. Exploration of photophysical properties involves extending the core pi-system with fused pyrene, phenanthrene, and naphthalene units, and varying electronic groups, where donor-acceptor systems across the core result in notably red-shifted emissions. Utilizing these fluorophores with redder emissions as organelle-targeting fluorescent tags in live cells showcases practical applications of the findings. Preliminary work has also been done in the incorporation of the azaphosphinines into larger, more complex receptors for complex oxyanions.
Key papers:
- J. N. McNeill, J. P. Bard, D. W. Johnson and M. M. Haley, Chem. Soc. Rev. 2023, 52, 8599-8634.
- J. P. Bard, S. G. Bolton, H. J. Howard, J. N. McNeill, L. N. Zakharov, D. W. Johnson, M. D. Pluth and M. M. Haley, J. Org. Chem. 2023, 88, 15516-15522.
- J. N. McNeill, M. A. Kascoutas, L. J. Karas, L. N. Zakharov, J. I.-C. Wu, D. W. Johnson and M. M. Haley, Chem. Eur. J. 2023, 29, e202203918.
- J. N. McNeill, L. J. Karas, J. P. Bard, K. Fabrizio, L. N. Zakharov, S. N. MacMillan, C. K. Brozek, J. I. Wu, D. W. Johnson and M. M. Haley, Chem. Eur. J. 2022, 28, e202200472.
- Bard, J. P.; McNeill, J. N.; Warren, G. I.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Isr. J. Chem. 2021, 61, 217-221.
- Bard, J. P.; Johnson, D. W.; Haley, M. M. Synlett. 2020, 31, 1862-1867.
- Bard, J. P.; Mancuso, J. L.; Deng, C.-L.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Supramol. Chem. 2020, 32, 49-55.
- Bard, J. P.; Bates, H. J.; Deng, C.-L.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. J. Org. Chem. 2019, 85, 85-91.
- Deng, C.-L.; Bard, J. P.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Org. Lett. 2019, 21, 6427-6431.
- Deng, C.-L.; Bard, J. P.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. J. Org. Chem. 2019, 84, 8131-8139.
- Bard, J. P.; Deng, C. L.; Richardson, H. C.; Odulio, J. M.; Barker, J. E.; Zakharov, L. N.; Cheong, P. H.-Y.; Johnson, D. W.; Haley, M. M. Org. Chem. Front. 2019, 6, 1257-1265.
- Takaesu, N. A.; Ohta, E.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Organometallics 2017, 36, 2491-2493.
- Vonnegut, C. L.; Shonkwiler, A. M.; Khalifa, M. K.; Zakharov, L. N.; Johnson, D. W.; Haley, M. M. Angew. Chem. Int. Ed. 2015, 54, 13318-13322.